Describe Jj Thomson Experiment on the Discovery of Electrons
British physicist JJ. Thomson is one of the most well-known physical experiments that led to electron discovery.
Discovery Of Electron Class 9 Structure Of An Atom
Thomson is the scientist that discovered electrons through an experiment called the Cathode Ray Experiment.

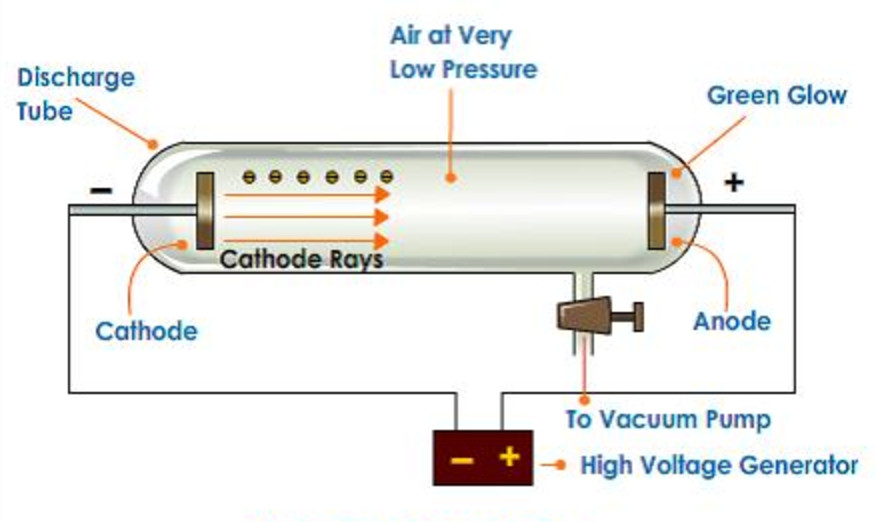
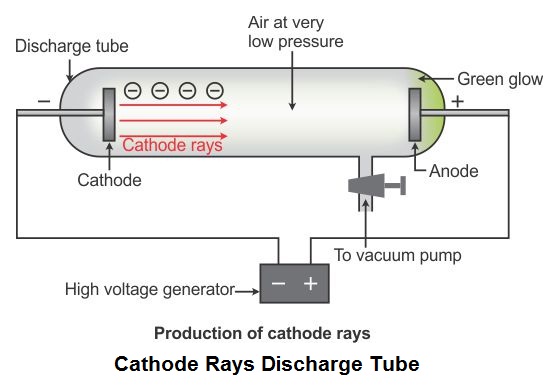
. Each generation benefits from the insights and discoveries of those who came before. Cathode ray tubes are sealed glass tubes from which most of the air has been evacuated. A cathode ray tube consists of two thin pieces of metals called electrodes sealed inside a glass tube with sealed ends.
He observed a greenish glow near the anode of the glass tube. Thomson announces the discovery of electrons On April 30 1897 British physicist JJ. Discovery of the electron - key takeaways In 1897 JJ Thomson discovered the electron by using cathode-ray tubes.
The rays which are emitted from the cathode hit the anode and cause the greenish glow. Describe JJ Thomson discovery - Used a series of cathode ray experiments to confirm the existence of the electron. JJ Thomsons experiment on cathode tubes eventually led to the discovery of the electron.
Rutherfords gold foil experiment showed that the atom is mostly empty space with a tiny dense positively-charged nucleus. This web exhibit ventures into the experiments by JJ. These negatively charged particles are an integral part of all atoms.
What is the cathode ray tube. Millikan oil drop experiment describe how conducted and conclusions - used electric field to suspend oil droplets. The experiment Cathode Ray Tube CRT conducted by J.
If I have seen a little further it is by standing on the shoulders of G. Thomsons 1897 experiments which helped bring understanding of. Both subjects were transformed by the experiments of J.
Thomson made a detailed study of the discharge of electricity through gases under very low pressure. Thomson began experimenting with cathode ray tubes. This meant that the ray itself was ___________________.
Cathode rays are nothing but a stream of negatively charged particles called electrons. The glass tube is attached to a vacuum pump and the pressure inside the tube is reduced to 001mm. The Cathode Ray Tube CRT experiment performed by J.
Along with the nearly contemporaneous discoveries of radioactivity and x rays the. When a high voltage is applied across the electrodes placed on the ends of the tube a beam flows from the negatively charged pole to the positively charged pole. This paper describes the simulation of J.
Metal electrodes are fitted to the ends of the glass tube as shown in the figure. It consists of a glass tube connected to two metal electrodes at two ends. Joseph John Thomson or JJ.
The scientist named JJ. Thomson is one of the most well-known physical experiments which led to the discovery of electrons. Electrons were the first of sub-atomic particles to be discovered by JJ.
Three big experiments that helped shape the electron and the atom concept that we have today are the discovery of the. Thomsons experiments with cathode ray tubes showed that all atoms contain tiny negatively charged subatomic particles or electrons. Thomson studied the characteristics and the constituents of cathode rays.
Cathode ray tubes are the glass tubes in which air is absent and in order to maintain this sealing is done. Brought to you by the American Institute of Physics. He created very low pressure inside the discharge tube and applied high voltage.
What were these particles called. Some Important Points Thomsons experiments were recognized and widely accepted and his cathode ray particles were known as electrons. The experiment could also describe characteristic properties essentially its affinity towards positive charge and its charge to mass ratio.
Thomson who in 1897 showed the existence of the charged particles that came to be known as electrons. This paper describes how J is simulated. He made use of electrically.
From his experiment Thomson arrived at the conclusion that. In the late century physicist JJ. Thomson that led to the discovery of a fundamental building block of matter.
This experiment was performed using a cathode ray tube Crookes tube. Late in the nineteenth century physicists were working hard to understand the properties of electricity and the nature of matter. -Thomson discovered that the cathode ray was made of particles.
Thomson invented to test the theory. The cathode ray tube was what JJ. Thomson and the discovery of the electron.
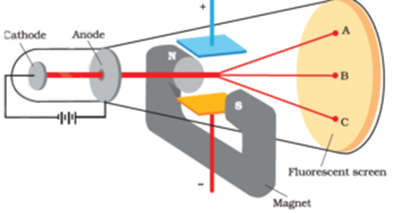
In addition the experiment could describe characteristic properties in essence its affinity to positive charge and its charge to mass ratio. Prior to the experiment it was not known that atoms were composed of. - When the ray was bent no electric charge could be detected at the other end.
Thomson put a magnetic field around the cathode ray tube. The gold foil experiment is one of the most important experiments. JJ Thomsons and others conducted an experiment to a vacuum pump.
The cathode ray discharge tube experiment performed by JJ. The charge of the electron was determined by an American scientist named Robert Millikan. Thomsons cathode ray tube experiment discovered the subatomic particle the electron.
An exhibit by the AIP Center for History of Physics with text animations and voice about JJ. Discovery of Electron. Up to 24 cash back 1st Experiment.
Thomson discovered the electrons by cathode ray experiment. He gave the proof of electrons in the atom by his experiment in which he had used cathode ray tubesThese tubes are a source of cathode rays production. Cathode ray tubes are essentially sealed vacuum chambers made up of glass.
Thereby allowing the electrostatic charge of the electron to be calculated. Thomson announced his discovery that atoms were made up of smaller components. Thomson led to the discovery of negatively charged particles called electron.
Discovery Of Electron Thomson S Experiment Milikan S Experiment

Describe J J Thomson S Experiment On The Discovery Of Electrons Brainly In
Describe J J Thomson 39 S Cathode Ray Experiment And How It Led To The Discovery Of Electrons What Are The Properties Of Electrons Chemistry Topperlearning Com N3yjj99

Who Discovered Electrons The Cathode Ray Experiment Selftution
0 Response to "Describe Jj Thomson Experiment on the Discovery of Electrons"
Post a Comment